Jordan university of science and technology faculty of engineering civil engineering department ce 453 environmental lab alkalinity acidity and determination of alkalinity in water experiment 4 student name.
Acidity and alkalinity of water experiment.
The key difference between acidity and alkalinity of water is that the acidity of water is the water s ability to neutralize a base whereas the alkalinity of water determines water s ability to neutralize an acid.
Total acidity or phenolphthalein acidity v 2 n 50 1000 sample vol 5 answer these questions.
Compared with the pond water the filtered drinkable water was almost acidic.
We can name acidity of water as the base neutralizing capacity and alkalinity of water as the acid neutralizing capacity.
The science behind water ph.
When the ph is above 7 0 the water is alkaline or basic there are more hydroxide ions than hydrogen ions.
Like acidity alkalinity is a net effect of the presence of several constituents but the most important are the bicarbonate hco3 carbonate co32 and hydroxyl oh anions.
You could add several pounds of 8 3ph sodium bicarb and make the same ph impact if you added a much smaller amount of 11 6 ph soda ash.
H 2 o co 2 h 2 co 3.
The use of acid injection sh ould be considered very carefully for several reasons.
So a water sample with a ph of 5 0 is ten times as acidic as one with a ph of 6 0.
A sample of water collected in the field had a ph of 6 8 which changed to 7 5 by the time the sample was brought to the laboratory.
We can determine these two parameters using.
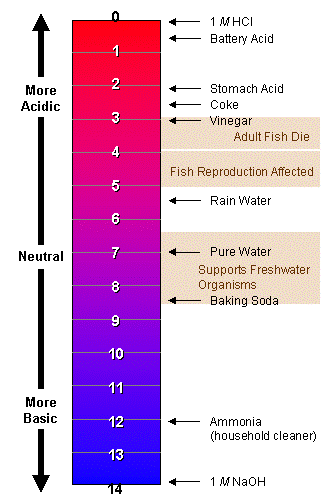
The ph scale is a measurement of how acidic or alkaline a substance is.
Ph 7 is neutral and will appear yellow on a litmus test strip.
Since co 2 controls the ph of the water you can either add co 2 directly to lower ph or you can use acid to convert bicarbonate alkalinity into carbonic acid h 2 co 3 which is just dissolved co 2.
Acidification of high alkalinity water.
Acidification of water having high ph but low alkalinity is rarely necessary.
Many greenhouse operators inject acid e g phosphoric nitric or sulfuric acid into water with problematic high levels of alkalinity.
Lab 4 alkalinity acidity and determination of alkalinity in water 1.
Anything orange to red is acidic while green to purple is basic.
Since the scale is logarithmic a drop in the ph by 1 0 unit is a 10 fold increase in acidity.
Alkalinity is determined by measuring how much standard acid must be added to a given amount of water in order to lower the ph to a specified value.